Current research goals
I am currently leading the following projects:
1. Overcoming Evasion from Sorafenib Treatment in Hepatocellular Carcinoma (HCC)
Sorafenib is the first systemic therapy approved for HCC. However, HCC rapidly evade sorafenib treatment, despite its multi-targeted activities. We are studying the pathways of evasion from sorafenib in HCC (P01/PPG Project 3). We are examining the role of MEK/ERK activation as a cell autonomous mechanism of escape. We are also studying how sorafenib-induced changes in HCC stroma lead to stromal-derived growth factor 1 alpha (SDF1-alpha/CXCL12)-mediated changes in vascular structure and function, inflammation/bone marrow-derived cell infiltration and fibrosis, and if these mediate HCC escape from treatment. To this end, we have developed orthotopic models of metastatic HCC in immunocompetent mice (Fig. 1), and have developed and acquired genetically engineered mouse models of HCC through our collaborations at MGH Cancer Center.
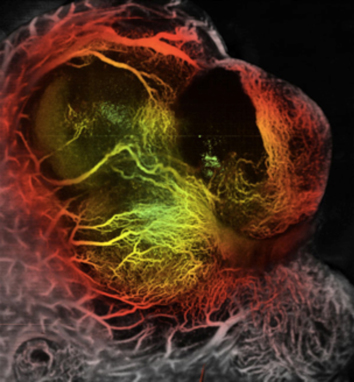
Fig: 1: Orthotopic HCA-1 tumor in C3H mouse liver. HCC vessels are visualized using Optical Frequency Domain Imaging.
In parallel, we are exploring the potential roles of stroma-derived factors in HCC patients treated with sorafenib and other antiangiogenic agents in collaboration with Dr. Zhu of the MGH Cancer Center.
2. Impact of SDF1-alpha Pathway Activation after Radiation Therapy on Local Tumor Control and Metastasis
We and others have found that SDF1-alpha/CXCR4 and HGF/cMET pathways can be activated by ionizing radiation in tumor stroma. Thus, we are currently evaluating the role of SDF1-alpha/CXCR4 or HGF/cMET inhibition as a sensitizer for radiation therapy in preclinical models. Specifically, we are examining 1) the role of SDF1-alpha and its receptors CXCR4 and CXCR7 in progression to metastasis after local radiotherapy (supported by an R01 grant); 2) the role of CXCR4 and bone marrow-derived cells in bone metastatic escape from palliative radiotherapy (funded through an ACS grant); and 3) the role of cMET inhibition in combination with radiotherapy in metastatic pancreatic adenocarcinoma models (supported by a Cummings Foundation grant). To this end, we have developed orthotopic models of prostate cancer, including a calvarial tumor model, which allows intravital microscopy imaging (Fig. 2) as well as orthotopic models of pancreatic adenocarcinoma.
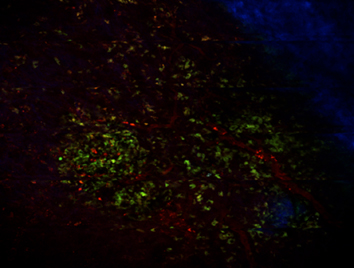
Fig. 2: Representative in vivo microscopy image of LNCaP-C2/GFP prostate cancer growing in the calvarium bone of nude mice. In green, prostate cancer cells; in red, functional tumor blood vessels; in blue, bone imaged by second harmonic generation by multiphoton microscopy.
In parallel, we are exploring the potential roles of SDF1-alpha/CXCR4 and HGF/cMET in prostate carcinoma, pancreatic adenocarcinoma, and HCC patients treated with radiotherapy in collaboration with MGH Cancer Center clinicians Drs. Efstathiou, Zhu and Hong (funded through NCI/Proton Beam Federal Share Program grants).
3. Circulating Biomarkers of Antiangiogenic Therapy
This project is carried out in the context of a large effort of clinical correlative studies (in over 25 clinical trials of antiangiogenic agents and/or radiotherapy), which include imaging, tissue and blood biomarker studies. This involves large teams of clinicians at MGH and Dana Farber Cancer Institute, and is funded through multiple collaborative grants from the NCI, DoD and NFCR. We are primarily focusing on the potential predictive biomarker value of soluble vascular endothelial growth factor (VEGF) receptor 1 (sVEGFR1 or sFLT1), as well as on the potential role of SDF1-alpha and HGF as biomarkers of escape from anti-VEGF therapies or radiotherapies (Fig. 3).